 Français
Français Antibiotic Drugs
Roxithromycin
Roxithromycin is a semi-synthetic long acting macrolide antibiotic.
Chemical structure
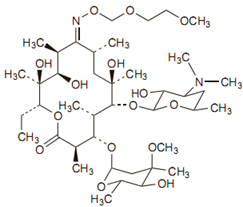
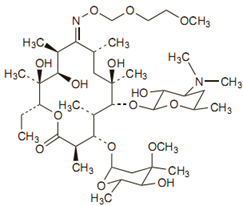
The empirical formula for roxithromycin is C41H76N2O15. Its molecular weight is 837.07. Its structure is:
Mechanism of action
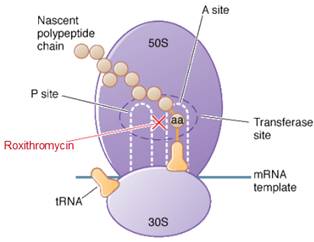
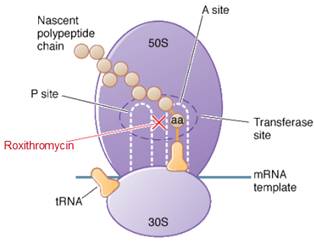
Roxithromycin is a bacteriostatic drug acts by inhibiting protein synthesis. It binds reversibly to 50S ribosomal subunits of sensitive microorganism. Roxithromycin interferes with transpeptidation and translocation thus there is inhibition of protein synthesis and hence inhibition of cell growth.
Site of action
Pharmacokinetics
Food interferes with absorption of roxythromycin so it should be taken on empty stomach or atleast 15 minutes prior to or 3 hours after meal. Peak plasma concentrations occur about 2 hours after a single dose of 150 mg.
Antimicrobial spectrum
Roxitromycin is effective against Streptococcus pyogenes, Streptococcus pneumoniae, Mycoplasma pneumoniae, Ureaplasma urealyticum, Chlamydia species, Moraxella catarrhalis, Gardenella vaginalis and Legionella.
Indication, administration and dosages
Roxithromycin is preferred for treating otitis media, sinusitis and pneumonia. It should be administered 15 minutes before food or on an empty stomach (i.e. more than 3 hours after a meal).
Adult: 150 mg bid or 300 mg once daily orally for 5-10 days in susceptible infections.
Child: 6-40 kg: 5-8 mg/kg daily.
Renal impairment: Dosage adjustment may be required.
Hepatic impairment: Usual daily doses should be halved in hepatic impairment.
Special Populations: Cirrhosis: 150 mg once daily.
Precautions, contraindications and warnings
Roxithromycin should be given with caution in patients with hepatic/renal impairment/QT prolongation. It is contraindicated in patients with known hypersensitivity to macrolides.
Adverse reactions
Side effects are rare and usually mild with roxithromycin. Nausea, vomiting, epigastric pain, diarrhoea, anorexia, flatulence, headache, dizziness, hypersensitivity may occur with roxythromycin.
Technical Description on Roxithromycin
Roxithromycin is a semi-synthetic long acting macrolide antibiotic.
Chemical structure
The empirical formula for roxithromycin is C41H76N2O15. Its molecular weight is 837.07. Its structure is:
Preparations available
Tablets - 150 mg/300 mg
Oral suspension - 50 mg/5 ml
Mechanism of action
Roxithromycin is bacteriostatic and inhibits protein synthesis by binding irreversibly to 50S ribosomal subunits of susceptible bacteria and other microorganisms, at or very close to the site that binds chloramphenicol. It does not inhibit peptide bond formation per se but rather inhibits the translocation step where a recently formed peptidyl tRNA molecule is transferred from the acceptor site (A) on the ribosome to the peptidyl donor site (P). Alternatively, macrolides may bind and cause a conformational change that terminates protein synthesis by indirectly interfering with transpeptidation and translocation. Thus there is inhibition of protein synthesis and hence inhibition of cell growth.Site of action
Microbiology
- Like erythromycin it is effective against Streptococcus pyogenes, Streptococcus pneumoniae, Mycoplasma pneumoniae, Ureaplasma urealyticum, and Chlamydia species.
- But is more potent against Moraxella catarrhalis, Gardenella vaginalis and Legionella and less potent against Bordetella pertussis.
- It is resistant to Enterobacteriaceae, Pseudomonas , Acinetobacter and multiresistant Staphylococcus aureus
Resistance
Resistance to macrolides including roxithromycin usually results from one of four mechanisms:
- ribosomal protection by inducible or constitutive production of methylase enzymes, mediated by expression of ermA, ermB, and ermC, which modify the ribosomal target and reduce drug binding (staphylococci, streptococci, Bacteroids fragilis, Corynebacterium diphtheriae, C. perfringens)
- drug efflux caused through an active pump mechanism (encoded by mrsA, mefA, or mefE in staphylococci, group A streptococci, or pneumococci respectively)
- macrolide hydrolysis caused due to esterases formed by Enterobacteriaceae
- chromosomal mutations that cause changes in a 50S ribosomal protein (found in B. subtilis, Campylobacter species, mycobacteria, pneumococci, Helicobacter pylori, Mycoplasma . pneumoniae, E. coli, Streptococcus pyogenes, and S. aureus)
Pharmacokinetics
The bioavailability of Roxithromycin after oral intake is about 50%. Absorption is reduced if taken after food so it should be taken at least 15 minutes before food or, otherwise, on an empty stomach i.e. more than 3 hours after having food. It attains maximal plasma concentrations after 2 hours of dosage administration. Roxithromycin has extensive tissue distribution. Roxithromycin is excreted during lactation in milk. The plasma protein binding is 96% and it principally binds to alpha1-acid glycoprotein. Roxithromycin is metabolized to various extents in the liver and mainly excreted via the faeces, urine and lungs. One of the principal metabolite is descladinose roxithromycin. The t1/2 is 8 to 13 hours.
Therapeutic indications
Uses are same like those of erythromycin but it is preferred for treating otitis media, sinusitis and pneumonia caused by Moraxella catarrhalis and pneumonia caused by legionella.
It should be administered 15 minutes before food or on an empty stomach (i.e. more than 3 hours after a meal).
In Adult persons: 150 mg twice daily or 300 mg OD orally for 5-10 days
In Children upto 6-40 kg: 5-8 mg/kg/day.
In Kidney dysfunction alteration in the dose may be necessary.
In patients of liver dysfunction the doses must be decreased to half the normal dose.
Drug Interactions
Roxithromycin has a much lesser affinity for cytochrome P450 isoenzymes than erythromycin and so has fewer interactions. It does not appear to interact with antacids, carbamazepine, oral contraceptives, prednisolone, or ranitidine.
Some interactions are:
- Roxithromycin increases effect of warfarin sodium, simvastatin, midazolam, digoxin, cyclosporine, cabergoline, acenocoumarol, and theophylline.
- Roxithromycin causes additive toxicity with amiodarone.
- It can displace disopyramide from protein binding sites.
Use in Children
It can cause neutropenia in children
It is recommended that the approved paediatric dose (i.e. 5 to 8 mg/kg/day for a maximum of 10 days) be sternly followed as higher doses can lead to bone plate growth abnormalities which have been seen in animal studies.
Use in Pregnancy (Category B1)
Animal studies have not shown any development abnormalities.
Lactation
Roxithromycin should be avoided in lactating mothers as minor amounts are excreted in the breast milk.
Carcinogenesis, Mutagenesis and Effects on Fertility
Animal studies have not proved on any of these.
Precautions
Roxithromycin should be given with caution in hepatic and renal impairment. Prolonged treatment increases risk of hepatotoxicity
Like other macrolides, roxithromycin can prolong the QT interval.
Like other macrolides, roxithromycin can aggravate myasthenia gravis.
Like other antibiotics it can lead to super-infections or pseudomembranous colitis.
Contraindications
- Prior history of hypersensitive reactions any of the macrolide antibiotics.
- Porphyria
- Liver dysfunction.
- Simultaneous treatment with ergot derived drugs.
Adverse reactions
Roxithromycin is usually well tolerated.
The following side-effects have been seen:
GIT
Vomiting, pain in the epigastrium, loose stools, decreased appetite
Hypersensitivity
Skin rashes, intense itching, angioedema and rarely, severe reactions can be seen like bronchospasm, anaphylaxis, laryngeal oedema, oedema all over the body and Stevens - Johnson syndrome.
Liver
Modest increase in serum transaminases, AST-ALT or ALP can be seen.