 Français
Français Antibiotic Drugs
Azithromycin
Azithromycin is a macrolide antibiotic.
Chemical structure
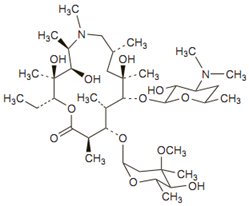
Its molecular formula is C38H72N2O12, and its molecular weight is 749.00. Azithromycin has the following structural formula:
Mechanism of action
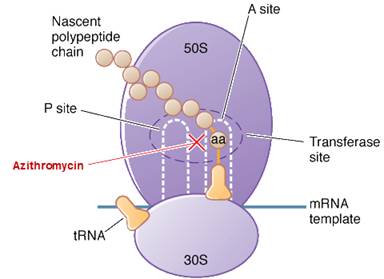
Azithromycin is a bacteriostatic drug acts by inhibiting protein synthesis. It binds reversibly to 50S ribosomal subunits of sensitive microorganism. Azithromycin interferes with transpeptidation and translocation thus there is inhibition of protein synthesis and hence inhibition of cell growth.
Site of action of azithromycin
Pharmacokinetics
Azithromycin is rapidly absorbed after oral administration. Food does not interfere with absorption of tablet or suspension of azithromycin but of capsule is reduced.
Peak plasma concentrations occur 2 to 3 hours after an oral dose and 1 to 2 hours after intravenous dosage.
Antimicrobial spectrum
Azithromycin has good activity against M. avium-intracellulare, Toxoplasma gondii, Cryptosporidium, Plasmodium species, H. influenza, Campylobacter species, M. catarrhalis, Chlamydia species, L. pneumophila, B. burgdorferi, Mycoplasma pneumoniae, Escherichia coli, Salmonella, Shigella species and H. pylori.
Indication, administration and dosage
Adults
| Condition | Dose |
| Any non-serious infection | 500 mg daily for 3 days OR 500 mg followed by 250 mg/day for next 4 days. |
| Uncomplicated genital infections (Chlamydia trachomatis) and chancroid | 1 g single dose |
| Uncomplicated gonorrhoea | 2 g single dose |
| Granuloma inguinale or lymphogranuloma venereum | 1 g followed by 500 mg daily, or 1 g/week for at least 3 weeks, until all lesions have completely healed. |
| Community-acquired pneumonia | 500 mg loading dose followed by 250-mg /day for next 4 days. |
| MAC infections | |
| Prophylaxis | 1.2 g once weekly |
| Treatment or secondary prophylaxis | 500 mg/day with other antimycobacterials |
Paediatric
| Condition | Dose |
| Acute otitis media or pneumonia | oral suspension 10 mg/kg on the first day (maximum: 500 mg) and 5 mg/kg (maximum: 250 mg per day) on days 2 through 5 |
| Otitis media (alternative therapy) | single 30 mg/kg dose |
| Tonsillitis or pharyngitis | 12 mg/kg per day, up to 500 mg total, for 5 days |
Precautions, contraindications and warnings
Azithromycin is contraindicated in patients with known hypersensitivity to azithromycin, erythromycin, any macrolide or ketolide antibiotic. It may cause QTc Prolongation, so avoid in patients with known QT prolongation.
Adverse reactions
Side effects with azithromycin are less frequent (<1%) and are usually mild. Dyspepsia, flatulence, vomiting, headache, somnolence, and taste disturbances are some of the side effects of azithromycin.
Technical Description on Azithromycin
Chemical structure
Azithromycin is an erythromycin derivative and its lactone ring contains an extra nitogen which is methylated. In most aspects it is similar to clarithromycin. These structural alterations improve acid stability and tissue penetration and widen the spectrum of activity.
Its molecular formula is C38H72N2O12, and its molecular weight is 749.00. Azithromycin has the following structural formula:

Mechanism of action
Azithromycin is bacteriostatic and like other macrolides inhibits synthesis of protein by binding reversibly to 50S ribosomal subunits of sensitive microorganisms, at or very close to the site that binds chloramphenicol. It does not inhibit peptide bond formation per se but rather inhibits the translocation step where a recently formed peptidyl tRNA travels from the acceptor site (A) on the ribosome to the peptidyl donor site (P). Alternatively, macrolides may bind and cause a conformational change that terminates protein synthesis by indirectly interfering with transpeptidation and translocation. Thus synthesis of proteins is inhibited.
At high concentrations azithromycin is bactericidal against susceptible strains. Cells are substantially more permeable to the unionized form of azithromycin, which possibly explains the augmented antimicrobial activity at alkaline pH.
Site of action of azithromycin

Microbiology
Azithromycin usually is less active than erythromycin against gram-positive organisms and slightly more active than either clarithromycin or erythromycin against H. influenzae and Campylobacter spp. It has good activity against M. catarrhalis, Chlamydia species, L. pneumophila, B. burgdorferi, Mycoplasma pneumoniae, Escherichia coli and shigella and salmonella species and H. pylori. It has enhanced activity against M. avium-intracellulare, Toxoplasma gondii, Cryptosporidium, and Plasmodium species. It is also more active than erythromycin against Chlamydia trachomatis and Ureaplasma urealyticum, and Mycobacterium avium complex.
Erythromycin-resistant gram-positive strains are cross resistant to azithromycin. E. faecalis and MRSA are also resistant to azithromycin. Beta-lactamase production does not affects azithromycin.
Resistance
Resistance to macrolides including azithromycin usually results from one of four mechanisms:
- ribosomal protection by inducible or constitutive production of methylase enzymes, mediated by expression of ermA, ermB, and ermC, which modify the ribosomal target and reduce drug binding (staphylococci, streptococci, B. fragilis, Clostridium perfringens, Corynebacterium diphtheriae, and Listeria and Legionella species)
- drug efflux by an active pump mechanism (encoded by mrsA, mefA, or mefE in staphylococci, group A streptococci, or S. pneumoniae, respectively)
- macrolide hydrolysis by esterases produced by Enterobacteriaceae
- chromosomal mutations that alter a 50S ribosomal protein (found in B. subtilis, Campylobacter species, mycobacteria, Str. pneumoniae, H. pylori, M. pneumoniae, Escherichia coli, Str. pyogenes, and Staph. aureus)
The most common type of resistance is a plasmid-mediated ability to methylate ribosomal RNA, resulting in decreased binding of the antimicrobial drug. This can lead to cross-resistance between erythromycin, other macrolides, lincosamides, and streptogramin B, as they share a common binding site on the ribosome and this pattern of resistance is known as the MLSB phenotype.
Incidence of resistance to azithromycin and other macrolides is higher among penicillin-resistant strains than among penicillin-sensitive strains
Preparations available
Tablets and capsules - contain azithromycin dihydrate 250 mg/500 mg
Oral suspension -contains azithromycin dihydrate powder 300 mg, 600 mg, 900 mg, or 1200
Azithromycin for injection – 500 mg/10 ml vial
Pharmacokinetics
After oral intake, azithromycin is rapidly absorbed, about 40% bioavailable and widely distributed. Absorption from capsules, but not tablets or suspension, is decreased by food. Peak plasma concentrations occur two to three after an oral dose and 1 to 2 hours after intravenous dosage. Concentrations are higher in tissues than in blood. It concentrates in WBCs, phagocytes and fibroblasts which contribute to drug distribution to inflamed tissues. Diffusion into the CSF is less when the meninges are not inflamed. Protein binding is 50% at very low plasma concentrations and less at higher concentrations. Animal studies indicate that it crosses the placenta. The antimicrobial activity of azithromycin is pH related and reduces with decreasing pH. It undergoes some hepatic metabolism to inactive metabolites, but it undergoes predominantly biliary excretion. About 6-12% of an oral dose is excreted unchanged in urine. The elimination t1/2 is 40-68 hours as it is slowly released from tissues.
Indications
Adults:
- Chlamydia pneumoniae acquired pneumonia, Mycoplasma pneumonia, pneumococci, or H. influenzae.
- Acute exacerbations of COPD due to bacterial infections and acute bacterial sinusitis due to H. influenzae, Moraxella catarrhalis or pneumococci.
- Chlamydia trachomatis or Neisseria gonorrhoeae induced urethritis and cervicitis.
- In men, genital ulcer due to Haemophilus ducreyi (chancroid).
- Skin and soft tissue infections (uncomplicated) due to Staphylococcus aureus, Streptococcus pyogenes, or Streptococcus agalactiae.
- Prophylaxis, and as a component of regimens in treatment of Mycobacterium avium complex (MAC) infections.
- Prophylaxis of endocarditis in at-risk patients unable to take penicillin.
- Azithromycin has been tried in protozoal infections such as babesiosis, crytosporidiosis, and toxoplasmosis.
- Trachoma and typhoid.
Paediatric Patients:
- Community-acquired pneumonia due to Chlamydia pneumoniae, H.influenzae, Mycoplasma pneumoniae or pneumococci.
- Acute otitis media caused by Haemophilus influenzae, Moraxella catarrhalis or Streptococcus pneumoniae.
Dosage
Capsule should be given at least one hour prior to or at least two hours after meals.
Adults
| Condition | Dose |
| Any non-serious infection | 500 mg daily for 3 days OR500 mg followed by 250 mg/day for next 4 days. |
| Uncomplicated genital infections (Chlamydia trachomatis) and chancroid | 1 g single dose |
| Uncomplicated gonorrhoea | 2 g single dose |
| Granuloma inguinale or lymphogranuloma venereum | 1 g followed by 500 mg daily, or 1 g/week for at least 3 weeks, until all lesions have completely healed. |
| Community-acquired pneumonia | 500 mg loading dose followed by 250-mg /day for next 4 days. |
| MAC infections | |
| Prophylaxis | 1.2 g once weekly |
| Treatment or secondary prophylaxis | 500 mg/day with other antimycobacterials |
Paediatric
| Condition | Dose |
| Acute otitis media or pneumonia | oral suspension 10 mg/kg on the first day (maximum: 500 mg) and 5 mg/kg (maximum: 250 mg per day) on days 2 through 5 |
| Otitis media (alternative therapy) | single 30 mg/kg dose |
| Tonsillitis or pharyngitis | 12 mg/kg per day, up to 500 mg total, for 5 days |
Drug interactions
- As azithromycin has a 15-member (not 14-member) lactone ring, it does not inactivate cytochrome P450 enzymes and therefore minimal interactions occur as compared with erythromycin and clarithromycin.
- Aluminium and magnesium antacids do not alter bioavailability but retard absorption and decrease peak serum concentrations of azithromycin, hence it should be given at least 1 hour before or 2 hours after the antacid
- Nelfinavir can increase plasma levels of azithromycin.
- Azithromycin can increase the therapeutic effect of oral anticoagulants.
Carcinogenesis, Mutagenesis, Impairment of Fertility, teratogenic effects: Studies in animals have not demonstrated any of these.
Contraindications and Warnings
- It is contraindicated in patients with prior history of hypersensitive reactions to any of the macrolides and in persons with a history of cholestatic jaundice/liver dysfunction associated with prior use of azithromycin.
- It must not be prescribed in patients with cystic fibrosis, generalized bacterial infection or severely ill patients.
- Clostridium Difficile-associated diarrhea can occur with its use.
- Can cause QT Prolongation – hence must not be given in patients with known QT interval prolongation, patients with proarrhythmic conditions like uncorrected or hypomagnesaemia or hypokalemia, decreased heart rate, and with antiarrthymics like quinidine, procainamide, dofetilide, amiodarone, sotalol.
- It can lead to exacerbation of symptoms of myasthenia gravis and new onset of myasthenic syndrome.
Adverse reactions
Gastrointestinal disturbances are the most frequent side effect of azithromycin but are usually mild and less frequent than with erythromycin. Headache, somnolence, and taste disturbances may occur.
Rarely seen side effects are:
GIT: vomiting, gastritis, diarrhoea, decreased appetite, constipation, dyspepsia, flatulence.
CVS: Palpitations, sometime pain in chest.
Genitourinary: vaginitis and nephritis.
CNS: Giddiness, convulsions, headache, vertigo, nervousness and increased sleepiness.
Allergic: Rashes, itching, photosensitivity.
Hematologic – Anemia and decreased WBC count.
General: Fever, fatigue, oedema.