 Français
Français Antibiotic Drugs
Ciprofloxacin
It is the most potent first generation bactericidal fluoroquinolone active against a wide range of bacteria.
Chemical structure
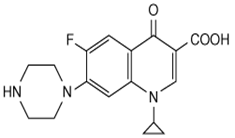
Its empirical formula is C17H18FN3O3 and MW is 331.3.The chemical structure is:
Mechanism of action
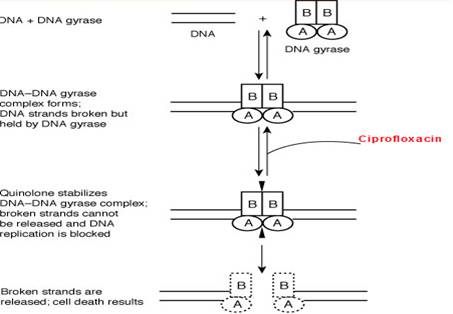
Ciprofloxacin inhibits the enzyme bacterial DNA gyrase and prevents replication of bacterial DNA during bacterial growth and reproduction.
Site of action
Pharmacokinetics
Ciprofloxacin is well absorbed after oral administration. Food delays its absorption. Blood concentrations of intravenously administered drug are similar to those of orally administered drug.
Antimicrobial spectrum
Ciprofloxacin is active against many gram-positive bacteria and gram-negative bacteria. Ciprofloxacin has rapidly bactericidal activity and high potency. Relatively long post-antibiotic effect. Protective intestinal streptococci and anaerobes are spared.
Indications, administration and dosage
| Condition | Severity | Dosage |
| Urinary Tract infection | Acute uncomplicated | 250 mg 12 hourly for 3 days |
| Mild/moderate | 250 mg 12 hourly for 7-14 days | |
| Severe/complicated | 500 mg 12 hourly for 7-14 days | |
| Chronic bacterial prostatitis | Mild/moderate | 500 mg 12 hourly for 28 days |
| Lower Respiratory Tract Infection | Mild/moderate | 500 mg 12 hourly for 7-14 days |
| Severe/complicated | 750 mg 12 hourly for 7-14 days | |
| Acute Sinusitis | Mild/moderate | 500 mg 12 hourly for 10 days |
| Skin and skin structure infection | Mild/moderate | 500 mg 12 hourly for 7-14 days |
| Severe/complicated | 750 mg 12 hourly for 7-14 days | |
| Bone and joint infection | Mild/moderate | 500 mg 12 hourly for > 4-6 weeks |
| Severe/complicated | 750 mg 12 hourly for > 4-6 weeks | |
| Intra-abdominal infections | Complicated | 500 mg 12 hourly for 7-14 days |
| Infectious Diarrhea | Mild/Moderate/Severe | 500 mg 12 hourly for 5-7 days |
| Typhoid fever | Mild/moderate | 500 mg 12 hourly for 10 days |
| Urethral and Cervical | Uncomplicated | 250 mg single dose |
| Inhalation anthrax (post exposure) | Bioterrorism agent | 500 mg 12 hourly for 60 days |
Precautions, contraindications and warnings
Dose modification is needed in patients of renal impairment. Caution in paediatric, geriatric, pregnant and nursing patients. Ciprofloxacin is contraindicated in patients with known hypersensitivity to ciprofloxacin or any other quinolone and in myasthenia gravis. Concomitant administration with tizanidine is contraindicated.
Adverse effects
Side effects are usually mild necessitates withdrawal only in 1.5%. Side effects include nausea, vomiting, bad taste, anorexia, dizziness, headache, restlessness, anxiety, insomnia, rash, pruritus, photosensitivity, urticarial, tendonitis and tendon rupture, hepatic and kidney toxicity.Technical Description on Ciprofloxacin
It is the most potent first generation fluoroquinolone effective against a number of bacteria.
Chemical structure
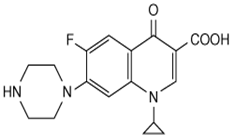
Ciprofloxacin is a quinolone antibiotic with one fluoro substitution. Its molecular formula is C17H18FN3O3 and MW is 331.3.The chemical structure is:
Preparations available
Oral: 100, 250, 500, 750 mg tablets; 500, 1000 mg extended-release tablet; 50, 100 mg/mL suspension
Parenteral: 10 mg/mL for IV infusion
Ophthalmic : 3 mg/mL solution; 3.3 mg/g ointment
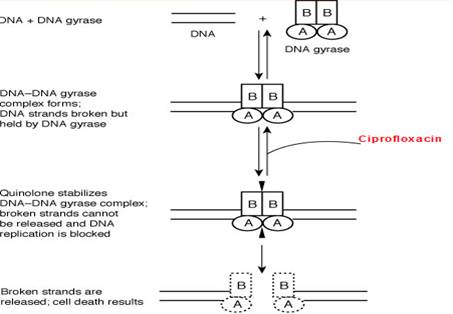
Mechanism of action
Ciprofloxacin like other fluoroquinolones (FQs) inhibits the enzyme bacterial DNA gyrase that produces cuts in the double-stranded DNA, leading to negative supercoiling and then re-ligation of the cut ends. This helps in averting positive supercoiling which may occur in excess. The DNA gyrase consists of two A and two B subunits: The A subunit brings about cutting of DNA, the B subunit causes negative supercoiling and then the A subunit causes resealing. Ciprofloxacin binds to A subunit with great affinity and restricts the nicking and resealing action. In gram-positive bacteria the major target of action is an analogous enzyme topoisomerase IV which nicks and separates daughter DNA strands once the DNA replication is complete. Ciprofloxacin is more potent against gram positive bacteria due to higher affinity for topoisomerase IV. The damaged DNA leads to formation of exonucleases resulting in digestion of the DNA and this possibly contributes to the bactericidal action of ciprofloxacin.
The mammalian cells possess an enzyme topoisomerase II instead of DNA gyrase or topoisomerase IV that has very low affinity for ciprofloxacin - thus the low toxicity to host cells.
Microbiology
Ciprofloxacin is active against:
Gram-positive bacteria
Staphylococcus aureus
Enterococcus faecalis
Staphylococcus epidermidis and saprophyticus
Pneumococci
Streptococcus pyogenes
Gram-negative bacteria
- Proteus (vulgaris and mirabilis)
- Campylobacter
- Citrobacter freundii
- Providencia (stuartii and rettgeri)
- E. coli, Klebsiella pneumoniae
- Haemophilus influenzae and parainfluenzae
- P. aeruginosa
- Salmonella typhi
- Neisseria gonorrhoeae
- Serratia marcescens
- Shigella species
- Moraxella catarrhalis
- Morganella morganii
The prominent microorganism which are resistant are: Bacteroides fragilis, Clostridia, anaerobic cocci.
The noteworthy microbiological features of ciprofloxacin are:
- Ciprofloxacin has highly potent action with swift bactericidal action.
- Plasmid resistant mutants are not easily selected.
- Anaerobes and streptococci present in the intestine which are useful are not affected.
- It is effective against the bacteria which are not sensitive to the aminoglycosides and beta lactam group of antibiotics.
- The bactericidal action is very less at decreased pH.
Resistance
Resistance is mainly because of chromosomal mutation forming a DNA gyrase or topoisomerase IV which has reduced affinity for ciprofloxacin. Another common mechanism is reduced permeability/increased efflux of ciprofloxacin across bacterial membranes. Like other FQs ciprofloxacin, FQ-resistant mutants are not easily selected hence resistance develops slowly to FQs. However, increasing resistance has been reported among Salmonella, Pseudomonas, staphylococci, gonococci, pneumococci and C. jejuni.
Due to the unique mechanism of action plasmid mediated transferable resistance perhaps does not occur.
A Qnr protein has been seen that offers protection to the DNA gyrase from damage by the FQs. Also modification of ciprofloxacin can be caused by a different type acetyltransferase which is similar to the one which modifies aminoglycoside.
Cross resistance is seen with the FQs.
Pharmacokinetics
After oral intake, the FQs have good oral absorption. Food delays absorption. It is extensive tissue distribution. The plasma t1/2 is about 3 to 5 hours. Plasma protein binding is 20-35%. It is excreted primarily in urine, both by glomerular filtration and tubular secretion. Urinary and biliary concentrations are10-50 folds higher than plasma. Plasma concentrations of orally given dose match with those of an IV given dose.
Therapeutic uses and Dosage
| Condition | Severity | Dosage |
| Urinary system infection | Acute uncomplicated | 250 mg 12 hourly for 3 days |
| Mild or moderate | 250 mg 12 hourly for 7-14 days | |
| Severe or complicated | 500 mg 12 hourly for 7-14 days | |
| Chronic prostate infection | Mild or moderate | 500 mg 12 hourly for 28 days |
| Infection of Lower Respiratory Tract | Mild or moderate | 500 mg 12 hourly for 7-14 days |
| Severe or complicated | 750 mg 12 hourly for 7-14 days | |
| Acute infection of the sinuses | Mild or moderate | 500 mg 12 hourly for 10 days |
| Skin infection | Mild or moderate | 500 mg 12 hourly for 7-14 days |
| Severe or complicated | 750 mg 12 hourly for 7-14 days | |
| Infection of bones and joints | Mild or moderate | 500 mg 12 hourly for > 4-6 weeks |
| Severe or complicated | 750 mg 12 hourly for > 4-6 weeks | |
| Urinary and reproductive tract infection | Uncomplicated | 250 mg single dose |
| Inhalation anthrax (post exposure) | 500 mg 12 hourly for 60 days | |
| Diarrhea (infectious) | Mild or Moderate or Severe | 500 mg 12 hourly for 5-7 days |
| Enteric fever | Mild or moderate | 500 mg 12 hourly for 10 days |
Drug Interactions
- Plasma concentration of theophylline, caffeine and warfarin are increased by ciprofloxacin due to inhibition of metabolism: toxicity of these drugs can occur.
- NSAIDs may augment the CNS toxicity of ciprofloxacin; seizures are reported.
- Antacids, sucralfate and iron salts given concomitantly decrease absorption of FQs.
- Concomitant administration of omeprazole reduces plasma concentration of ciprofloxacin.
- Both reduced and enhanced serum levels of phenytoin may be seen in persons taking simultaneous Ciprofloxacin.
- Concurrent prescription of Ciprofloxacin with glyburide has, rarely can lead to severe hypoglycaemia.
- The excretion of ciprofloxacin through the kidney is affected negatively by probenecid leading to enhanced serum levels of ciprofloxacin.
- The oral absorption of ciprofloxacin is enhanced by metoclopramide.
- Ciprofloxacin increases the serum levels of ropinirole, lidocaine, sildenafil and clozapine.
- Excretion of methotrexate by the kidney is inhibited by ciprofloxacin.
- Precaution should be taken when using Ciprofloxacin concomitantly with class IA or III antiarrhythmics as Ciprofloxacin may have an additive effect on the QT interval
Special populations
Renal Impairment
Alteration of the dosage regimen is necessary for patients with impairment of renal function
Elderly population
Ciprofloxacin can lead to enhanced risk of developing severe tendon disorders including tendon rupture which is enhanced in persons taking corticosteroids.
Children
Ciprofloxacin, like other quinolones, causes arthropathy and histological changes in large joints which bear the weight of juvenile animals resulting in lameness
Pregnancy
It is a Category C drug. There is not much data available about the effect of ciprofloxacin in pregnancy.
Lactation
Ciprofloxacin is secreted during lactation.
Warning and precautions
- Rarely crystalluria can be seen in individuals taking ciprofloxacin. Alkalinity of the urine should be avoided in persons taking ciprofloxacin and they should be well hydrated to prevent the formation of highly concentrated urine.
- Like other FQs, there are increased chances of inflammation of tendon and even rupture of tendons due to ciprofloxacin.
- Like other FQs, ciprofloxacin can lead to deterioration of weakness of muscles in patients of myasthenia gravis.
- Hypersensitive reactions can be seen with ciprofloxacin which can be fatal rarely.
- Clostridium difficile-associated enterocolitis can be seen with ciprofloxacin.
- There are increased chances of arthropathy and tendon rupture has been observed in children.
- Rarely large or small sized neurons may be affected by polyneuropathy due to ciprofloxacin leading to altered sensations.
- CNS effects like agitative behaviour, decreased sleep, anxiousness and vivid dreams can be seen with ciprofloxacin.
- Photosensitive reactions can be seen with ciprofloxacin.
Carcinogenic potential, mutagenic effect and impaired fertility
Animal studies have not shown any of these effects.
Contraindications
Ciprofloxacin is contraindicated in persons with a history of hypersensitivity to Ciprofloxacin or any member of the quinolone class of antimicrobial agents.
Concomitant administration with tizanidine is contraindicated.
Adverse effects
The commonly observed adverse effects are:
- GIT: vomiting, altered taste , decreased appetite
- CNS: giddiness, headache, anxious behaviour, restlessness, decreased sleep, impairment of concentration, tremor. Seizures are rare; occur only at high doses or when predisposing factors are present.
Other rare reactions are:
- Hypersensitive: skin rashes, increased itching ,photosensitivity
- Tendonitis and tendon rupture
- Vasculitis, joint pain , muscle pain
- Allergic pneumonitis;
- Acute kidney insufficiency or failure;
- Hepatitis; jaundice; acute necrosis of liver or failure;
- Anemia, including haemolytic and aplastic; decreased platelet count, thrombotic thrombocytopenic purpura; decreased WBC count, agranulocytosis; pancytopenia.