 Français
Français Antibiotic Drugs
Clarithromycin
Clarithromycin is a macrolide antibiotic like erythromycin.
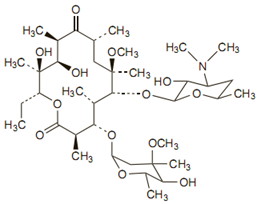
Chemical structure
The molecular formula is C38H69NO13, and the molecular weight is 747.96.
Mechanism of action
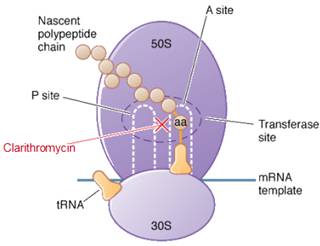
Clarythromycin is a bacteriostatic drug acts by inhibiting protein synthesis. It binds reversibly to 50S ribosomal subunits of sensitive microorganism. Clarythromycin interferes with transpeptidation and translocation thus there is inhibition of protein synthesis and hence inhibition of cell growth.
Site of action of azithromycin
Pharmacokinetics
Clarythromycin is rapidly absorbed orally and food does not interfere with its absorption. Peak plasma concentrations occur 2 to 3 hours after an oral dose.
Pharmacokinetics of clarithromycin are altered in patients with hepatic or renal dysfunction.
Antimicrobial spectrum
Clarithromycin has enhanced activity against sensitive strains of streptococci and staphylococci, Moraxella catarrhalis, Legionella species, Chlamydia trachomatis, B. burgdorferi, Mycoplasma pneumoniae, H. pylori and Ureaplasma urealyticum, some protozoa, Mycobacterium avium complex, M. leprae.
Clarithromycin enhance the activity of antimycobacterials like isoniazid, rifampicin, pyrazinamide and ethambutol against Mycobacterium tuberculosis.
Indications, administration and dosage
Adults
| Indication | Dose |
| Respiratory-tract infections, otitis media, skin and soft-tissue infections. | 250 mg twice daily or 500 mg twice daily in severe infection for 5-7 days |
| H. pylori eradication in peptic ulcer | 500 mg twice daily with another antibacterial or PPI or H2receptor antagonist, for 7 to 14 days. |
| Leprosy | 500 mg daily |
| Prophylaxis and treatment of nontuberculous mycobacterial infections | 500 mg twice daily |
The usual IV dose is 500 mg twice daily, as an infusion over 60 minutes using a solution containing about 0.2% of clarithromycin for 2 to 5 days.
Children - Oral dose is 7.5 mg/kg twice daily and in > 12 years of age - usual adult dose.
Precautions, contraindications and warning
Clarithromycin is contraindicated in patients with a known hypersensitivity to Clarithromycin, erythromycin, or any macrolide. Clarithromycin should not be given to patients with history of QT prolongation. Avoid in pregnant women.
Adverse reactions
Side effects are generally mild and less frequent with clarithromycin. Diarrhea, nausea, abnormal taste, dyspepsia, abdominal pain/discomfort may occur with clarithromycin. Intravenous doses may cause phlebitis and pain at the injection site.
Technical Description on Clarithromycin
It is a macrolide antibiotic similar to erythromycin.
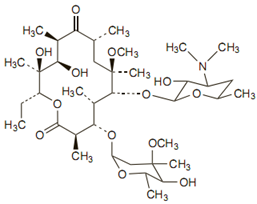
Chemical structure
Clarithromycin differs from erythromycin only by methylation of the hydroxyl group at the 6 position. Due to which it has improved acid stability and oral absorption compared with erythromycin. Chemically, it is 6-0 -methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96.
Mechanism of action
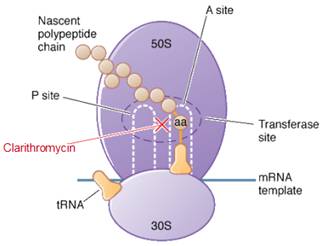
Clarithromycin is bacteriostatic and like other macrolides leads to inhibition of protein synthesis by binding reversibly to 50S ribosomal subunits of susceptible bacteria or other microorganisms, at or very close to the site that binds chloramphenicol. It does not inhibit peptide bond formation per se but rather inhibits the translocation step where a recently formed peptidyl tRNA molecule is transferred from the acceptor site (A) on the ribosome to the peptidyl donor site (P). Alternatively, macrolides may bind and can lead to a conformational alteration which stops protein synthesis by indirectly interfering with transpeptidation and translocation. Thus synthesis of protein is inhibited leading to hinderance in the growth of cell.
Site of action
Pharmacokinetics
Clarithromycin is swiftly absorbed after oral intake, and the bioavailability is about 50-55%. Food has not much effect on the oral absorption. Peak plasma concentrations occur 2 to 3 hours after an oral dose. The active metabolite of clarithromycin i.e. 14-hydroxyclarithromycin and clarithromycin, distribute widely and attains achieve good concentration in the intracellular sites. Plasma protein binding is about 40-80%. Clarithromycin is excreted during lactation. Clarithromycin undergoes extensive hepatic metabolism, and about 5-10 % is excreted in faeces and about 20-40% is excreted unchanged in the urine. 14-Hydroxyclarithromycin as well as other metabolites is also excreted in the urine, accounting for 10 to 15% of the dose. The t1/2 of clarithromycin and its active metabolite14-hydroxyclarithromycin are about three to seven and five to nine hours respectively. Metabolism is saturable, resulting in nonlinear pharmacokinetics with higher dosages leading to higher t1/2. The alteration of dosage is not required in liver or kidney dysfunction.
Microbiology
- Clarithromycin is marginally more potent than erythromycin against sensitive strains of streptococci and staphylococci, Moraxella catarrhalis, Legionella species, Chlamydia trachomatis, B. burgdorferi, Mycoplasma pneumoniae, H. pylori and Ureaplasma urealyticum.
- It has modest activity against H. influenzae, H. parainfluenzae and N. gonorrhoeae.
- Erythromycin-resistant streptococci and staphylococci are also resistant to clarithromycin.
- Clarithromycin has enhanced activity against some protozoa like Toxoplasma gondii, Cryptosporidium, and Plasmodium.
- Clarithromycin is more active than erythromycin or azithromycin against Mycobacterium avium complex and M. leprae.
- The major metabolite, 14-hydroxy clarithromycin, is also active, and enhances the activity of clarithromycin notably against H. influenzae.
- Beta-lactamase production should have no effect on Clarithromycin activity.
- Clarithromycin has been reported to enhance the activity of a numerous antimycobacterials including ethambutol, isoniazid, pyrazinamide, and rifampicin against Mycobacterium tuberculosis.
Resistance
Resistance to macrolides including clarithromycin usually results from one of four mechanisms:
- ribosomal protection by inducible or constitutive production of methylase enzymes, mediated by expression of ermA, ermB, and ermC, which modify the ribosomal target and reduce drug binding (staphylococci, streptococci,B. fragilis, Clostridium perfringens, Corynebacterium diphtheriae, and Listeria and Legionella species)
- drug efflux caused due to the presence of an active pump mechanism which is encoded by mrsA, mefA, or mefE in staphylococci, group A streptococci, or pneumococci , respectively)
- macrolide hydrolysis caused by esterases present in Enterobacteriaceae .
- chromosomal mutations that modify a 50S ribosomal protein which is present in B. subtilis, mycobacteria, pneumococci, Campylobacter species,H. pylori, M. pneumoniae, Escherichia coli, Str. pyogenes, and S. aureus)
There is a decrease in the binding of clarithromycin due to methylation of the ribosomal RNA which is mediated by plasmids. This is the commonest variety of resistance to clarithromycin As a result of this there can be cross resistance between other macrolides like erythromycin, lincosamides, and also streptogramin B. This is due to the fact that all the above mentioned antibiotics have a mutual binding region on the ribosome and this type of resistance is labelled as the MLSB phenotype.
Incidence of resistance to clarithromycin and other macrolides is higher among penicillin-resistant strains than among penicillin-sensitive strains.
Clarithromycin-resistant isolates of H. pylori have also developed. Genetic mutations responsible for clarithromycin resistance have been identified in H. pylori and in Mycobacterium. As resistance develops swiftly in M. avium during clarithromycin monotherapy, combination therapy is recommended.
Uses and Dosage
Adults
| Indication | Dose |
| Respiratory-tract infections (including otitis media) and skin and soft-tissue infections. | 250 mg twice daily or 500 mg twice daily in severe infection for 5-7 days |
| H. pylori eradication in peptic ulcer | 500 mg twice daily with another antibacterial or PPI or H2receptor antagonist, for 7 to 14 days. |
| Leprosy | 500 mg daily |
| Prophylaxis and treatment of non-tuberculous mycobacterial infections | 500 mg twice daily |
The usual IV dose is 500 mg twice daily, as an infusion over 60 minutes using a solution containing about 0.2% of clarithromycin for 2 to 5 days.
Children - Oral dose is 7.5 mg/kg twice daily and in > 12 years of age - usual adult dose.
Other uses:
It can be used for prophylaxis of endocarditis as an alternative to penicillins.
It is used with pyrimethamine as an alternative regimen in the treatment of toxoplasmosis.
Contraindications
- Clarithromycin is contraindicated in persons with a prior history of hypersensitive reactions to any of the macrolide antibiotics.
- Previous history of cholestatic jaundice or liver dysfunction occurring due to clarithromycin is also a contraindication.
- QT prolongation or ventricular arrhythmias are a contraindication to the use of clarithromycin.
- Clarithromycin in combination with ranitidine bismuth citrate is contraindicated in patients with a history of acute porphyria.
Warnings
- Clarithromycin has been shown to be teratogenic in animals and should be avoided in pregnancy.
- Like other antibiotics, Clostridium difficile associated diarrhea (CDAD) can be seen with Clarithromycin.
Drug Interactions
- Kidney or liver impairment is a contraindication for simultaneous prescription of colchicine and clarithromycin.
- Due to increased probability of myopathy and rhabdomyolysis the statins (HMG-CoA reductase inhibitors) particularly those metabolized by CYP3A4 must not be prescribed along with Clarithromycin.
- Clarithromycin is contraindicated with drugs that prolong the QT interval like the antihistaminics like astemizole, terfenadine and other drugs like cisapride and pimozide.
- Hypotension occurs if clarithromycin is given with verapamil, diltiazem or the dihydropyridine amlodipine. All these calcium channel blockers undergo metabolism through CYP3A4.
- Substantial hypoglycaemia can occur due to simultaneous prescription of oral hypoglycemics or insulin and clarithromycin as it leads to inhibition of CYP3A enzyme.
- Prominent increase in prothrombin time and INR is seen when warfarin and clarithromycin are given together.
- Simultaneous prescription of zidovudine with clarithromycin can lead to reduced plasma concentration of zidovudine.
- Digoxin is a known substrate for P-glycoprotein (Pgp) and Clarithromycin inhibits Pgp. When Clarithromycin and digoxin are co-administered, inhibition of Pgp by Clarithromycin may lead to increased exposure of digoxin.
- Clarithromycin increases the plasma levels of carbamazepine and theophylline.
- Clarithromycin will be metabolized more by CYP3A inducers like rifampicin, rifabutin, rifapentine (antitubercular drugs) and protease inhibitors like Efavirenz and Nevirapine. Whereas the concentration of 14-OH-Clarithromycin is increased.
- The metabolism of sildenafil and other phosphodiesterase inhibitors like vardenafil and tadalafil is inhibited by clarithromycin as it inhibits the CYP3A enzyme which metabolizes the phosphodiesterase inhibitors.
- Clarithromycin inhibits CYP3A which increases the levels of the benzodiazepines like alprazolam, triazolam and midazolam.
- Itraconazole and clarithromycin are metabolized by CYP3A, hence simultaneous prescription can lead to interactions which can proceed in any direction.
Precautions
In severe kidney impairment dosage of clarithromycin should be halved or the dosing interval doubled.
Clarithromycin can lead to deterioration of signs and symptoms of myasthenia gravis
Adverse Effects
- Gastrointestinal disturbances (diarrhea, nausea, abnormal taste, dyspepsia, abdominal pain), are the most frequently seen but are generally mild and less frequent with clarithromycin than with erythromycin.
- Smell and taste disturbances, stomatitis, glossitis, tongue and tooth discoloration.
- Transient CNS effects (mostly headache).
- Arthralgia, myalgia, hypoglycaemia, leucopenia, and thrombocytopenia.
- Interstitial nephritis and renal failure have been reported rarely.
- Intravenous doses may cause phlebitis and pain at the injection site.