 Français
Français Antibiotic Drugs
Linezolid
Linezolid is a synthetic oxazolidinone class of antibiotic.
Chemical structure
The empirical formula is C16H20FN3O4. Its molecular weight is 337.35, and it’s chemical Structure is:
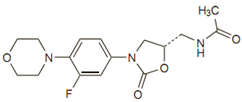
Mechanism of action
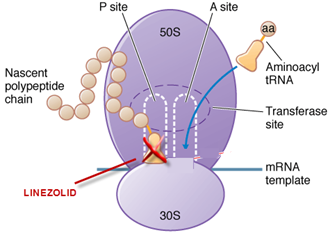
Linezolid inhibits protein synthesis by binding to the P site of the 50S ribosomal subunit and preventing formation of the larger ribosomal complex. Linezolid stops protein synthesis before it begins. Due to its unique action linezolid is active against strains that are resistant to multiple other agents.
Site of action
Pharmacokinetics
Linezolid is very well absorbed orally virtually 100%, so its oral and injectable dosage are same. Peak concentration is achieved in 1-2 hours.
Antimicrobial spectrum, uses, administration and dosage
| Condition | Dosage -Adults | Dosage - Children (upto 11 years) | Duration of Treatment |
| Vancomycin-resistant E. faecium infection | 600 mg twice daily | 10 mg/kg IV or oral 8 hourly | 14-28 days |
| Nosocomial pneumonia caused by methicillin-susceptible S. aureus and MRSA | 600 mg twice daily | 10 mg/kg IV or oral 8 hourly | 10-14 days |
| Community-acquired pneumonia caused by penicillin-susceptible strains of Strep. pneumoniae | 600 mg twice daily | 10 mg/kg IV or oral 8 hourly | 10-14 days |
| Complicated skin and skin structure infections caused by streptococci and methicillin-susceptible S. aureus and MRSA | 600 mg twice daily | 10 mg/kg IV or oral 8 hourly | 10-14 days |
| Uncomplicated skin and skin-structure infections | 400 mg twice daily | <5 yrs: 10 mg/kg oral 8 hourly 5-11 yrs: 10 mg/kg oral 12 hourly | 10-14 days |
It should be reserved as an alternative agent for treatment of infections caused by multiple-drug-resistant strains. It should not be used when other agents are likely to be effective. In-judicious use and overuse will hasten selection of resistant strains.
Precautions, contraindications and warnings
Contraindicated in patients who have known hypersensitivity to linezolid and in patients taking any drug which inhibits monoamine oxidases A or B (e.g. phenelzine, isocarboxazid), directly and indirectly acting sympathomimetic agents (e.g. pseudoephedrine), vasopressive agents (e.g. epinephrine, norepinephrine), dopaminergic agents (e.g. dopamine, dobutamine) and in patients with uncontrolled hypertension, pheochromocytoma, thyrotoxicosis.
Monitor platelet counts in patients with risk of bleeding if linezolid taken beyond 2 weeks.
Adverse reactions
Myelosuppression, including anemia, leukopenia, and pancytopenia, reversible thrombocytopenia, diarrhoea, constipation, nausea, vomiting, headache, pruritis, rash, peripheral neuropathy, optic neuritis, and lactic acidosis may occur with linezolid.
Technical Description on Linezolid
Linezolid is a synthetic antimicrobial agent belonging to the class known as oxazolidinone.
Chemical structure
The molecular formula is C16H20FN3O4. Its MW is 337.35, and it’s chemical
structure is as given below :
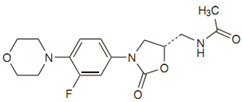
Preparations available
Oral: 400,600 mg tablets; 100 mg powder for 5 mL suspension
Parenteral: 2 mg/mL for IV infusion
Mechanism of Action
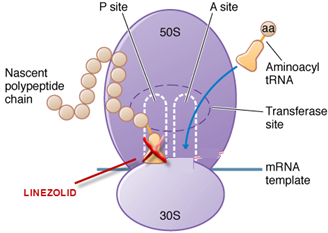
Linezolid inhibits protein synthesis due to binding to the P site of the 50S ribosomal subunit and preventing formation of the larger ribosomal ternary N-formyl methionine –tRNA (-fMet-tRNA)-70S complex that initiates protein synthesis. Binding of linezolid distorts the t-RNA binding site overlapping both 50S and 30S ribosomal subunits and stops protein synthesis before it begins. Therefore there is no cross resistance with other antibacterials. Because of its unique mechanism of action, linezolid is active against strains that are resistant to multiple other agents.
Site of action
Antimicrobial Activity
Linezolid is effective against gram + ve micro-organisms like staphylococci including Methicillin resistant staphylococci aureus (MRSA), Vancomycin resistant staphylococci aureus (VRSA) penicillin resistant streptococcus pyogens, streptococcus viridans and streptococcus pneumonia, enterococci including Vancomycin resistant enterococci (VRE), gram-positive anaerobic cocci, Clostridia, Bacteroides fragilis, Bacillus anthracis, Corynebacterium species and Listeria monocytogenes. Linezolid is bactericidal against streptococci, pneumococci and B. fragilis and bacteriostatic against enterococci and staphylococci. It has poor activity against most gram-negative aerobic or anaerobic bacteria as it is effluxed out by them. Mycobacterium tuberculosis is moderately susceptible.
Resistance
Point mutations of the 23S rRNA leads to resistance in enterococci and staphylococci. As multiple copies of 23S rRNA genes exist in bacteria, resistance usually requires mutations in two or more copies.
Pharmacokinetics
Linezolid is well absorbed orally and food does not interfere with its absorption. As oral bioavailability is virtually 100%, dosing for oral and intravenous preparations is similar. Peak serum concentrations are achieved 1-2 hours after a single 600-mg dose in adults. The t1/2 is ~4-6 hours. Post antibiotic effect lasts for 1.5 hours. Linezolid is 30% protein-bound and distributes extensively to well-perfused tissues. It undergoes non-enzymatic oxidation to form aminoethoxyacetic acid and hydroxyethyl glycine derivatives. About 80% of the dose appears in the urine, 50% as the two primary oxidation products and 30% as active compound. 10 % of the dose appears as oxidation products in faeces. Linezolid and its oxidation products are removed by dialysis; so the drug should be given after haemodialysis.
Therapeutic Uses and Dosage
| Condition | Dosage –Adults | Dosage - hildren(up to 11 years) | Duration of Treatment |
| Vancomycin-resistant E. faecium infection | 600 mg twice daily | 10 mg/kg IV or oral 8 hourly | 14-28 days |
| Methicillin-susceptible S. aureus and MRSA dependent nosocomial pneumonia. | 600 mg twice daily | 10 mg/kg IV or oral 8 hourly | 10-14 days |
| Community-acquired pneumonia due to penicillin-susceptible strains of Strep. pneumoniae | 600 mg twice daily | 10 mg/kg IV or oral 8 hourly | 10-14 days |
| Streptococci, methicillin-susceptible S. aureus and MRSA dependent skin structure infections and complicated skin | 600 mg twice daily | 10 mg/kg IV or oral 8 hourly | 10-14 days |
| Skin infections and uncomplicated skin. | 400 mg twice daily | <5 yrs: 10 mg/kg oral 8 hourly 5-11 yrs: 10 mg/kg oral 12 hourly | 10-14 days |
- Linezolid is bacteriostatic for staphylococci and enterococci; it should not be used as first-line therapy for treatment of suspected endocarditis.
- It should be reserved as an alternative agent for treatment of infections caused by multiple-drug-resistant strains. It should not be used when other agents are likely to be effective. In-judicious use and overuse will hasten selection of resistant strains.
Drug Interactions
- Linezolid is neither a substrate nor an inhibitor of CYPs hence interactions are minimal.
- Linezolid is a weak, reversible monoamine oxidation inhibitor.
- So if linezolid is co-administered with serotonergic, dopaminergic or adrenergic drugs, most frequently selective serotonin reuptake inhibitor antidepressants, it can lead to serotonin syndrome (confusion, hypertension, seizures, tachycardia, muscle rigidity and even death).
- It can also result in “cheese reaction” if food or beverages containing tyramine are consumed. Avoid dietary tyramine ≥100 mg per meal.
- Concurrent use with tramadol may increase risk of seizures.
- Avoid concurrent use or use within 2 wk of stopping another MAO Inhibitor to reduce risk of hypertensive crisis which can be fatal.
- Linezolid causes additive toxicity with dapoxetine and desvenlafaxine.
Drug-Laboratory Test Interactions
There are no reported drug-laboratory test interactions.
Contraindications
- Linezolid is contraindicated in patients who have known hypersensitivity to linezolid.
- Monoamine Oxidase Inhibitors - Linezolid is contraindicated in patients taking any drug which inhibits monoamine oxidases A or B (e.g. phenelzine, isocarboxazid) or within two weeks of taking any such drug.
- Linezolid is contraindicated in patients with uncontrolled hypertension, pheochromocytoma, thyrotoxicosis and/or patients taking any of the following types of medications: directly and indirectly acting sympathomimetic agents (e.g. pseudoephedrine), vasopressive agents (e.g. epinephrine, norepinephrine), dopaminergic agents (e.g. dopamine, dobutamine)
- It is contraindicated in patients with carcinoid syndrome and/or patients taking any of the following medications: serotonin re-uptake inhibitors, tricyclic antidepressants, serotonin 5-HT1 receptor agonists (triptans), meperidine or buspirone.
Precautions
Platelet counts should be scrutinized in patients with risk of bleeding, pre-existing thrombocytopenia or intrinsic or acquired disorders of platelet function and in patients receiving courses of therapy lasting beyond 2 weeks.
Nursing Mothers - Linezolid and its metabolites are excreted in the milk of lactating rats. It is not known whether linezolid is excreted in human milk. Caution should be exercised when it is administered to a nursing woman.
Pregnancy - Pregnancy Category C – No significant teratogenic effects were found in animal studies. It should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus.
Carcinogenesis, Mutagenesis, Impairment of Fertility – animal studies have shown no significant effect on any of these parameters.
Adverse Effects
Haematological Toxicity
- Myelo-suppression, including anemia, leukopenia, and pancytopenia has been reported.
- Reversible thrombocytopenia has been seen in 2.4% of treated patients, and its occurrence is related to duration of therapy.
Other effects
- Diarrhoea, constipation, nausea, vomiting, headache, pruritis and rash.
- Taste alteration, oral/vaginal candidiasis, tongue discolouration
- Dizziness, insomnia, convulsions, abnormal liver function tests, fever.
- Patients receiving long-term (>8 weeks) linezolid can develop peripheral neuropathy, optic neuritis, and lactic acidosis which may originate from effects of linezolid on the mitochondria (mitochondrial 70S ribosomes).