 Français
Français Antibiotic Drugs
Cefixime
Cefixime is a third generation oral bactericidal cephalosporin.
Chemical structure
The chemical formula is C16H15N5O7S2, 3H2O and MW is 507.50 as the trihydrate. The structure is:
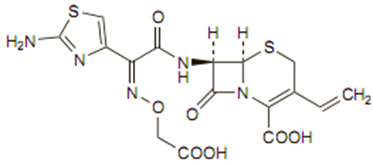
Mechanism of action
Mechanism of action of cefixime is similar to penicillin. Cefixime acts by inhibiting bacterial cell wall synthesis. Lack of bacterial cell wall results in death due to lysis of bacteria.
Pharmacokinetics
Food does not interfere with extent of absorption of cefixime but it delays time required to attain peak plasma concentration. Average peak concentrations produced by oral suspension are approximately 25%-50% higher than the tablets. Tablets should not be replaced for oral suspension in the treatment of otitis media due to lack of bioequivalence.
Antimicrobial spectrum
Cefixime is extremely stable in the presence of β-lactamase enzymes hence many organisms resistant to penicillins and some cephalosporins may be susceptible to cefixime. Cefixime is highly active against gram negative cocci, gram negative bacilli and anaerobes than gram positive cocci and bacilli. It is not active against pseudomonas.
Indications and usage
Cefixime is used in treatment of uncomplicated urinary tract infections, otitis media, acute bronchitis, acute exacerbation of chronic bronchitis, uncomplicated gonorrhoea.
Administration and dosage
| Condition | Adults | Children |
| All indications | 400 mg daily | 8 mg/kg/day |
The recommended formulation in children is suspension. Children weighing more than 50 kg or older than 12 years should be treated with the recommended adult dose of cefixime. In the treatment of infections due to S. pyogenes, the treatment should be for at least 10 days.
Precautions, contraindications and warnings
Cefixime is contraindicated in patients with known allergy to the cephalosporin group of antibiotics. Cefixime should be used during pregnancy only if clearly needed. Dose reduction needed in patients with renal impairment.
Adverse reaction
Side effects of cefixime includes diarrhea, loose stools, abdominal pain, dyspepsia, nausea, vomiting, pseudomembranous colitis, hypersensitivity reactions, hepatic toxicity, renal toxicity, headaches, dizziness and seizures.
Technical Description on Cefixime
Cefixime is a third generation oral cephalosporin.
Chemical structure
Like other cephalosporins it consists of a dihydrothiazine ring fused to a beta-lactam ring containing an appropriate side chain at position 7. The chemical formula is C16H15N5O7S2, 3H2O and MW is 507.50 as the trihydrate. The structure is: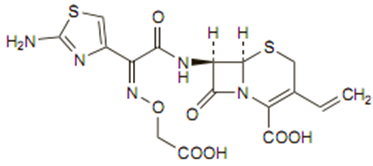
Preparations available
Oral: 200, 400 mg tablets; powder for oral suspension, 100 mg/5 mL
Mechanism of action
Like other cephalosporins, cefixime possesses a mechanism of action similar to penicillins i.e. inhibition of transpeptidation process resulting in the formation of imperfect cell wall; osmotic drive from the outside isotonic environment of the host cell to the inside of the hypertonic bacterial cytoplasm and finally activation of the autolysin enzyme leading to the lysis of bacteria. Thus cefixime is also a bactericidal drug.
Microbiology
Cefixime is active against:
Gram-positive
- Streptococcus pneumoniae,
- Streptococcus pyogenes.
Gram-negative Organisms
- E.coli
- H. influenzae
- Moraxella catarrhalis
- Proteus mirabilis
- N. gonorrhoeae
The following organisms are resistant to cefixime:
- Group D streptococci (which includes enterococci)
- Listeria monocytogenes
- Staphylococcal strains (which includes )
- Enterobacter species
- Pseudomonas species
- Bacteroides fragilis
- Clostridia
Resistance
Resistance to cefixime like other cephalosporins is due to:
- Failure of the antibiotic in reaching the active site
- Modifications in the penicillin-binding proteins (PBPs)
This can lead to the binding of the cephalosporin to Beta –lactamases which hydrolyzes the Beta -lactam ring and inactivates the cephalosporin.
Streptococcus pneumoniae become resistant to the cephalosporins of the 3rd generation by alterations in the 2 penicillin binding proteins (1A & 2X).
Destruction by hydrolysis of beta lactam structure is the most predominant way of resistance to cefixime like other cephalosporins. Enormous amounts of Beta-lactamase are released by many gram positive organisms. The gram negative bacteria produce decreased amounts of the beta lactamase enzyme but its periplasmic position makes them more capable of destructing the cephalosporins.
Pharmacokinetics
After oral intake cefixime undergoes about 40%-50% absorption. But, when administered with food time to maximal absorption is increased about 0.8 hours. It has a plasma t1/2 of 3–4 hours. Peak serum concentrations reach between 2 and 6 hours after oral administration. Average peak concentrations produced by oral suspension are approximately 25%-50% higher than the tablets. Tablets should not be replaced for oral suspension in the treatment of otitis media due to lack of bioequivalence.
Cefixime is not metabolized significantly. It is both excreted in the urine and eliminated in the bile. About 50% is excreted in urine without any metabolism within one day. The serum protein binding is approximately 65%. Cefixime is not cleared significantly from the blood by haemodialysis or peritoneal dialysis.
Therapeutic uses
Cefixime is indicated for treatment of the following conditions:
- Infections of the urinary system which are not complicated due to proteus mirabilis and E. coli.
- Otitis Media caused by Streptococcus pyogenes, H. influenzae and Moraxella catarrhalis.
- Urethral or cervical gonorrhoea which is not complicated due to N. gonorrhoeae.
- Acutely developed exacerbations of Chronic Bronchitis and acutely developed Bronchitis due to H. influenzae and pneumococci.
Dosage
| Condition | Adults | Children |
| All indications | 400 mg daily | 8 mg/kg/day |
- The recommended formulation in children is suspension.
- Children weighing > 50 kg or > 12 years of age must be prescribed with the adult dose.
- The oral suspension must be used to treat otitis media.
Drug Interactions
- Simultaneous prescription of cefixime with anticoagulants like warfarin may lead to increase in the prothrombin time.
- Carbamazepine: Cefixime leads to increase in plasma levels of carbamazepine when administered concomitantly.
Interactions with laboratory tests
- A false-positive direct Coombs test may be seen.
- Tests for ketones which use nitroprusside can lead to false-positive result.
- Urine glucose estimation with Benedict’s solution or Fehling’s solution can lead to false positive result if cefixime is used simultaneously.
Mutagenicity, carcinogenic potential and impaired fertility
Short term animal studies and in vitro studies have not shown any carcinogenic or mutagenic activity or impairment of fertility with cefixime.
Pregnancy
It is a category B drug and animal studies have not shown any teratogenic effect.
Nursing Mothers
Distribution of cefixime in human milk is not known.
Paediatric population
It has not been studied in children < 6 months old.
Renal Impairment
The dose should be adjusted in accordance with the severity of the renal impairment.
CONTRAINDICATIONS
Cefixime is contraindicated in patients with known allergy to the cephalosporin group of antibiotics.
ADVERSE REACTIONS
The commonly observed adverse effects are:
- GIT: Vomiting, pain in the abdomen, loose stools, dyspepsia, and rarely pseudomembranous colitis.
- Kidney: Acute renal failure, increased creatinine or blood urea nitrogen.
- Hematopoietic system: decreased platelet count, decreased WBC count particularly involving neutrophils and eosinophils.
- Liver – hepatitis, increased AST, ALT and ALP which may be transient, and Hyperbilirubinemia.
- Allergic Reactions: rashes on the skin, anaphylaxis, increased itching, generalized oedema, and rarely Stevens-Johnson syndrome.
CNS: Convulsions, giddiness