 Français
Français Antibiotic Drugs
Ofloxacin
Ofloxacin is a synthetic, broad spectrum first generation bactericidal fluoroquinolone.
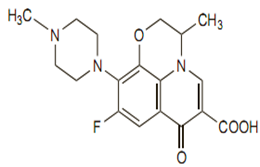
Chemical structure
Its empirical formula is C18H20FN3O4, and its molecular weight is 361.4.The chemical structure is:
Mechanism of action
Ofloxacin inhibits the enzyme bacterial DNA gyrase and prevents replication of bacterial DNA during bacterial growth and reproduction.
Pharmacokinetics
Nearly 100% of the orally administered dose is absorbed. Food delays rate of absorption of ofloxacin but do not significantly affect the extent of absorption.
Antimicrobial spectrum
Ofloxacin is active against many gram-positive bacteria and gram-negative bacteria. Relatively long post-antibiotic effect. Ofloxacin has intermediate activity between norfloxacin and ciprofloxacin for gram-negative bacteria. It has better activity against gram-positive organism and further better against chlamydia and mycoplasma compared to ciprofloxacin.
Indications, administration and usage
| Condition | Dosage (adults) |
| Acute Bacterial Exacerbation of Chronic Bronchitis | 400 mg 12 hourly for 10 days |
| Comm. Acquired Pneumonia | 400 mg 12 hourly for 10 days |
| Uncomplicated Skin and Skin Structure Infections | 400 mg 12 hourly for 10 days |
| Acute, Uncomplicated Urethral and Cervical Gonorrhoea | 400 mg single dose |
| Non-gonococcal Cervicitis/Urethritis due to C. trachomatis | 300 mg 12 hourly for 7 days |
| Mixed Infection of the urethra and cervix due to C. trachomatis and N. gonorrhoeae | 300 mg 12 hourly for 7 days |
| Acute Pelvic Inflammatory Disease | 400 mg 12 hourly for 10-14 days |
| Uncomplicated Cystitis due to E. coli or K. pneumonia | 200 mg 12 hourly for 3 days |
| Uncomplicated Cystitis due to other approved pathogens | 200 mg 12 hourly for 7 days |
| Complicated UTI's | 200 mg 12 hourly for 10 days |
| Prostatitis due to E.Coli | 300 mg 12 hourly for 6 weeks |
Precautions, contraindications and warnings
Patients receiving ofloxacin should be well hydrated to prevent the formation of highly concentrated urine so that crystalluria does not occur. Ofloxacin is contraindicated in patients with known hypersensitivity to ofloxacin or any other fluoroquinolone and in patients with QTc prolongation.
Adverse reaction
Side effects of norfloxacin are dizziness, nausea, headache, abdominal cramps, anorexia, diarrhea, constipation, dyspepsia, flatulence, tingling of the fingers, vomiting, tendonitis, tendon repture, liver toxicity and kidney toxicity.
Technical Description on Ofloxacin
Ofloxacin is a synthetic, broad spectrum first generation fluoroquinolone.
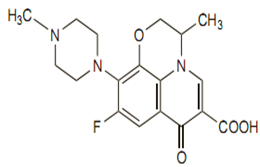
Chemical structure
Its empirical formula is C18H20FN3O4, and its molecular weight is 361.4.The chemical structure is:
Preparations available
Oral: 200, 300, 400 mg tablets
Ophthalmic : 3 mg/mL solution
Otic : 0.3% solution
Mechanism of action
Ofloxacin like other fluoroquinolones (FQs) inhibits the enzyme bacterial DNA gyrase that nicks double-stranded DNA, introduces negative supercoils and then reseals the nicked ends. This is essential to avert excessive positive supercoiling of the strands when they separate to permit replication or transcription. The DNA gyrase consists of two A and two B subunits: The A subunit brings about nicking of DNA, B subunit introduces negative supercoils and then A subunit reseals the strands. Ofloxacin binds to A subunit with high affinity and restricts its strand cutting and resealing function. In gram-positive bacteria the major target of action is an analogous enzyme topoisomerase IV which nicks and separates daughter DNA strands once the DNA replication is complete. The damaged DNA leads to formation of exonucleases resulting in digestion of the DNA and this possibly contributes to the bactericidal action of ofloxacin.
The mammalian cells possess an enzyme topoisomerase II instead of DNA gyrase or topoisomerase IV that has very low affinity for ofloxacin - thus the low toxicity to host cells.
Microbiology
Aerobic gram-positive microorganisms
- Staphylococcus aureus (methicillin-susceptible strains)
- Streptococcus pneumoniae (penicillin-susceptible strains)
- Streptococcus pyogenes
Aerobic gram-negative microorganisms
- Citrobacter (diversus) koseri
- Enterobacter aerogenes
- Escherichia coli
- Haemophilus influenzae
- Klebsiella pneumoniae
- Neisseria gonorrhoeae
- Proteus mirabilis
- Pseudomonas aeruginosa
Like other fluoroquinolones, some strains of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with ofloxacin.
Other microorganisms
- Chlamydia trachomatis
Ofloxacin is not active against Treponema pallidum.
Various strains of other streptococcal species, Enterococcus species, and anaerobes are resistant to ofloxacin.
Resistance
Like other fluoroquinolones, resistance is mainly because of chromosomal mutation (Quinolone-Resistance Determining Regions {QRDRs}) forming a DNA gyrase or topoisomerase IV with reduced affinity for ofloxacin. Another common mechanism is reduced permeability/increased efflux of ofloxacin across bacterial membranes. Like other FQs ofloxacin, FQ-resistant mutants are not easily selected hence resistance develops slowly to FQs.
Due to the unique mechanism of action of fluoroquinolones plasmid mediated transferable resistance perhaps does not occur.
Recently two types of plasmid-mediated resistance have been reported.
- The first type utilizes Qnr proteins, which protect DNA gyrase from the fluoroquinolones.
- The second is a variant of an aminoglycoside acetyltransferase capable of modifying ofloxacin.
Both mechanisms confer low-level resistance which may facilitate the point mutations that confer high-level resistance. Resistance to one fluoroquinolone, particularly if it is of high level, usually confers cross-resistance to all other members of the class.
Resistance to ofloxacin due to spontaneous mutation in vitro is rare.
Pharmacokinetics
Ofloxacin is rapidly and well absorbed orally and bioavailability is almost 100%.The peak plasma concentration is attained 1 to 2 hours after an oral doseof 400 mg. Absorption may be delayed by the presenceof food, but the extent of absorption is not substantiallyaffected. Plasma protein binding is approximately 25%. Ofloxacin isextensively distributed in body fluids, including the CSF,and tissue penetration is good. It crosses the placentaand is distributed into breast milk. It also appears in thebile. The elimination of ofloxacin is biphasic; half-lives ofabout 4 to 5 and 20 to 25 hours have been reported forthe 2 phases, respectively. In renal impairment, the t1/2 increases to about15 to 60 hours. There is limitedmetabolism to desmethyl and N-oxide metabolites and desmethylofloxacin has moderate antibacterial activity. Ofloxacin is excreted primarily by the kidneys by tubular secretion and glomerular filtration. About 65 to 80% of a dose is eliminated unchanged in theurine over 24 to 48 hours, resulting in high urinary concentrations. Less than 5% is excreted in the urine asmetabolites. About 4 to 8% of a dose may be eliminatedin the faeces.
Therapeutic uses and dosage
| Condition | Dosage (adults) |
| Acute Bacterial Exacerbation of Chronic Bronchitis | 400 mg 12 hourly for 10 days |
| Comm. Acquired Pneumonia | 400 mg 12 hourly for 10 days |
| Uncomplicated Skin and Skin Structure Infections | 400 mg 12 hourly for 10 days |
| Acute, Uncomplicated Urethral and Cervical Gonorrhoea | 400 mg single dose |
| Non-gonococcal Cervicitis/Urethritis due to C. trachomatis | 300 mg 12 hourly for 7 days |
| Mixed Infection of the urethra and cervix due to C. trachomatis and N. gonorrhoeae | 300 mg 12 hourly for 7 days |
| Acute Pelvic Inflammatory Disease | 400 mg 12 hourly for 10-14 days |
| Uncomplicated Cystitis due to E. coli or K. pneumoniae | 200 mg 12 hourly for 3 days |
| Uncomplicated Cystitis due to other approved pathogens | 200 mg 12 hourly for 7 days |
| Complicated UTI's | 200 mg 12 hourly for 10 days |
| Prostatitis due to E.Coli | 300 mg 12 hourly for 6 weeks |
All the conditions mentioned in the above table are due to susceptible organisms stated in the microbiology section
Drug interactions
- Cimetidine can interfere with the elimination of some quinolones including ofloxacin, though their interaction has not been studied.
- Elevated serum levels of cyclosporine have been reported with concomitant use of cyclosporine with some other quinolones. The potential for interaction between
ofloxacin and cyclosporine has not been studied.
- Quinolones, including ofloxacin, may enhance the effects of oral anticoagulants, including warfarin or its derivatives or similar agents.
- Quinolones including ofloxacin when given with glyburide can rarely lead to severe hypoglycaemia.
- Like other fluoroquinolones ofloxacin inhibits CYP1A2 in vitro. When used with other drugs metabolized by CYP1A2 like caffeine, clozapine, ropinirole, tacrine, theophylline, tizanidine it may lead to increased substrate drug concentrations when given in normal doses.
- Probenecid inhibits the renal excretion of ofloxacin thereby increasing its levels.
- Non-steroidal anti-inflammatory drug (NSAID) and ofloxacin given together may enhance the risk of CNS stimulation and convulsive seizures.
- As multivitamins, or other formulations containing iron or zinc, antacids, sucralfate and didanosine chewable/buffered tablets or the paediatric powder for oral solution interfere with the absorption of ofloxacin; they should not be given concurrently with, or within 2 hours of, the administration of norfloxacin.
- Use of fluoroquinolones including ofloxacin can lead to increase in plasma levels of theophylline when used concurrently.
Warnings and precautions
- Patients receiving ofloxacin should be well hydrated to prevent the formation of highly concentrated urine so that crystalluria does not occur.
- Like other fluoroquinolones, ofloxacin is associated with an enhanced risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
- Moderate to severe photosensitivity/phototoxicity reactions, can be associated with the use of quinolones including ofloxacin after sun or UV light exposure. Therefore, excessive exposure to these sources of light should be avoided.
- Central Nervous System Effects/Disorders: Convulsions have been reported in patients receiving ofloxacin. Convulsions, increased intracranial pressure (including pseudotumor cerebri), and toxic psychoses have been reported in patients receiving drugs in this class. Quinolones may also cause central nervous system (CNS) stimulation which may lead to tremors, restlessness, light-headedness, confusion, and hallucinations.
- Hypersensitivity Reactions: Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been seen in patients receiving quinolone therapy, including ofloxacin.
- Clostridium Difficile Associated Diarrhea: Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ofloxacin and may range in severity from mild diarrhea to fatal colitis.
- Peripheral Neuropathy: Rarely ofloxacin can lead to sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness.
- Ofloxacin should be not be used in patients with known prolongation of the QT interval, patients with uncorrected hypokalemia, and patients receiving class IA (quinidine, procainamide), or class III (amiodarone, sotalol) antiarrhythmic agents.
Special populations
- Geriatric
The frequency or severity of adverse reactions was not different in elderly adults compared with younger adults treated with ofloxacin. Also the pharmacokinetic properties were similar. Dosage adjustment is necessary for elderly patients with impaired renal function
- Pregnancy
It is a pregnancy Category C drug. Ofloxacin has not shown any teratogenic effect in most of the animal species tested. However well-controlled studies in pregnant women have not been conducted. Ofloxacin should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus.
- Nursing Mothers
Ofloxacin is excreted in human milk with concentration same as in plasma.Hence they should be avoided during lactation.
- Paediatric Use
The safety and effectiveness of oral ofloxacin in paediatric patients and adolescents below the age of 18 years have not been established. Ofloxacin causes arthropathy in juvenile animals of several animal species.
- Renal impairment
In patients with impaired renal function (creatinine clearance <50 mg/mL), modification of the dosage regimen is mandatory.
- Hepatic impairment
In patients with known or suspected hepatic insufficiency/impairment, careful clinical observation and appropriate laboratory studies should be performed before and during therapy since excretion of ofloxacin may be reduced.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Long-term carcinogenicity studies in rats and mice resulted in no carcinogenic or tumorigenic effects due to ofloxacin. Also no adverse effects were observed in reproduction studies.
Contraindications
Ofloxacin is contraindicated in persons with a history of hypersensitivity associated with the use of ofloxacin or any member of the quinolone group of antimicrobial agents.
Adverse effects
The commonly seen adverse effects are:
- Nausea, insomnia, headache, dizziness, diarrhea, vomiting, dysgeusia
- Rash, pruritus, external genital pruritus in women, vaginitis
- Hematopoietic: anemia, leukopenia, leukocytosis, neutropenia, neutrophilia, increased band forms, lymphocytopenia, eosinophilia, lymphocytosis, thrombocytopenia, thrombocytosis, elevated ESR
- Hepatic: elevated: alkaline phosphatase, AST (SGOT), ALT (SGPT)
- Serum chemistry: hyperglycemia, hypoglycemia, elevated creatinine, elevated BUN
- Urinary: glucosuria, proteinuria, alkalinuria, hyposthenuria, hematuria, pyuria
Less commonly seen adverse effects were:
- Abdominal pain and cramps, chest pain, decreased appetite, dry mouth
- Fatigue, flatulence, gastrointestinal distress, nervousness
- Pharyngitis, fever, sleep disorders, somnolence, trunk pain
- Vaginal discharge, visual disturbances, constipation