 Français
Français Antibiotic Drugs
Amoxicillin - Clavunate Potassium
Amoxicillin is extended spectrum penicillin and clavunate potassium is a β-lactamase inhibitor. Addition of clavunate potassium with amoxicillin further extends the antimicrobial spectrum of amoxicillin against β-lactamase producing bacteria.
Chemical structure
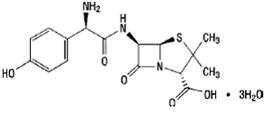
Amoxicillin
The molecular formula is C16H19N3O5S•3H2O and the molecular weight is 419.45. The chemical structure is:

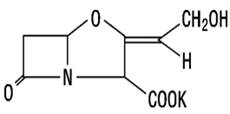
Clavunate potassium
The clavulanate potassium molecular formula is C8H8KNO5, and the molecular weight is 237.25. The chemically structure is:
Mechanism of action
Clavunate potassium is an irreversible inhibitor of β-lactamases produced by both gram-positive and gram-negative bacteria. It prevents hydrolysis of amoxicillin resulting in extending the spectrum of amoxicillin to β-lactamase producing bacteria. Clavuate potassium does not possess any significant antimicrobial action itself.
Pharmacokinetics
Amoxicillin and clavulanate potassium are well absorbed orally and also can be given parenterally.
Pharmacokinetics of amoxicillin and clavunate potassium are largely similar and neither appears to affect the other to any great magnitude.
Antimicrobial spectrum
Clavunate potassium is a potent inhibitor of β-lactamases produced by Gram-negative bacteria including Haemophilus ducreyi, H. influenzae, Neisseria gonorrhoeae, Moraxella catarrhalis, Bacteroides fragilis, and some Enterobacteriaceae. It also inhibits β-lactamases produced by S. aureus. Clavulanic acid reverses resistance to amoxicillin in beta-lactamase-producing strains of species otherwise sensitive and also augments the activity of amoxicillin against numerous species not normally considered sensitive.
Indications, administration and dosage
Amoxicillin - clvunate potassium combination is reserved for bacterial infections caused by amoxicillin-resistant beta-lactamase-producing strains.
Acute exacerbations of chronic bronchitis, cellulitis, empirical antibiotic therapy for hospital acquired infections are indication for use of this combination.
Neonates and infants aged <12 weeks (3 months): The recommended dose of Amoxicillin + Clavulanic acid is 30 mg/kg/day divided 12 hourly.
Patients > 3 months of age
| INFECTIONS | 12 hourly | 8 hourly |
| Otitis media, sinusitis, lower respiratory tract infections, and more severe infections | 45 mg/kg/day | 40 mg/kg/day |
| Less severe infections | 25 mg/kg/day | 20 mg/kg/day |
Patients Weighing > 40 kg and adults:
| INFECTIONS | 12 hourly | 8 hourly |
| Otitis media, sinusitis, lower respiratory tract infections, and more severe infections | 875 mg | 500 mg |
| Less severe infections | 500 mg | 250 mg |
Precautions, contraindications and warnings
Chances of superinfection should be kept in mind while prescribing amoxicillin - clavunate potassium. It is contraindicated in patients with a history of allergic reactions to any penicillin and patients of mononucleosis.
Adverse reactions
Diarrhea, nausea, skin rashes, urticaria, vomiting, vaginitis are frequently reported side effects with this combination. Rare side effect includes abdominal discomfort, flatulence, headache, and thrombocytosis. Risk of acute liver injury was about 6 times greater with the combination than with amoxicillin alone.
Technical Description on Amoxicillin - Clavunate Potassium
In this orally active antibiotic preparation amoxicillin has been combined with the beta-lactamase inhibitor clavulanate potassium. Addition of Clavunate potassium makes amoxicillin active against β-lactamase producing organisms.
Amoxicillin
It is an extended spectrum, penicillinase (β-lactamase)-susceptible, semi-synthetic amino-penicillin. It is a close chemical and pharmacological congener of ampicillin (amino-p-hydroxy-benzyl penicillin). Amoxicillin like other penicillins, inhibit the penicillin binding proteins (PBPs {specifically PBP-1A}), which are transmembrane surface enzymes that catalyse the cross linking (transpeptidation) between the peptidoglycans in the bacterial cell wall.
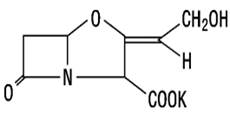
Clavulanic acid
Clavunate (Clavulanic acid) is obtained from cultures of Streptomyces clavuligerus. It has a beta-lactam structure similar to penicillin nucleus. It has very less intrinsic antimicrobial activity, but it attaches to numerous types of beta lactamases and inhibits them. The clavulanate potassium molecular formula is C8H8KNO5, and the MW is 237.25. The chemically structure is:
Pharmacokinetics
Clavulanic acid is well absorbed orally (75%) and also can be given parenterally. The plasma protein binding is 22-30% and it undergoes hepatic metabolism. Renal excretion accounts for > 50% and plasma half-life is 1 hour.
Mechanism of action
Clavulanic acid inhibits a various types of β-lactamases (class II to class V, but not class I cephalosporinase) produced by both gram-positive and gram-negative bacteria. It is a progressive inhibitor; binding withβ-lactamase is reversible initially but becomes covalent later-inhibition increasing with time. It is a 'suicide inhibitor’. It inactivates cell bound enzymes and permeates the outer layers of gram-negative bacteria cell wall and also inhibits the periplasmically located β-lactamase. The penicillin group of antibiotics and also cephalosporins are rendered ineffective by plasmid mediated and chromosome induced beta lactamases which is clinically important. Clavulanic acid is predominantly effective against such beta-lactamases.
It is a potent inhibitor of β-lactamases produced by Gram-negative bacteria including H. ducreyi, H. influenzae, Moraxella catarrhalis, N. gonorrhoeae and Bacteroides fragilis. It also inhibits β-lactamases formed by S. aureus. As clavulanic acid is not that active against type 1 beta-lactamases(chromosomally mediated); therefore, P. aeruginosa, many Citrobacter, Enterobacter, Serratia and Morganella species, and are not sensitive to it. It does not inhibit some extended-spectrum beta-lactamases (plasmid-mediated) in K. pneumoniae, other Enterobacteriaceae, and Pseudomonas aeruginosa.
Thus Clavulanic acid augments the activity of amoxicillin, other penicillins and cephalosporins against many resistant strains of bacteria.
Preparations available
Tablet - Amoxicillin 250 mg + Clavulanic acid 125 mg, Amoxicillin 500 mg + Clavulanic acid 125 mg, Amoxicillin 875 mg + Clavulanic acid 125 mg
Chewable tablet- 125 mg, 200 mg, 250mg and 400mg
Oral suspension - 125 mg/5 mL, 200 mg/5 mL, 250 mg/5 mL and 400 mg/5 mL
Dosage and administration
Doses of the combination are based on the amoxicillin content.
Neonates and infants aged <12 weeks (3 months): The recommended dose of Amoxicillin + Clavulanic acid is 30 mg/kg/day divided 12 hourly.
Patients > 3 months of age
| INFECTIONS | 12 hourly | 8 hourly |
| Otitis media, sinusitis, lower respiratory tract infections, and more severe infections | 45 mg/kg/day | 40 mg/kg/day |
| Less severe infections | 25 mg/kg/day | 20 mg/kg/day |
Patients Weighing > 40 kg and adults:
| INFECTIONS | 12 hourly | 8 hourly |
| Sinusitis, Otitis media, lower respiratory tract infections, and severe infections | 875 mg | 500 mg |
| Less severe infections | 500 mg | 250 mg |
Pharmacokinetics
Amoxicillin and clavulanate potassium are well absorbed orally. Clavunate is rapidly absorbed with a bioavailability of 60 % and it can be given parenterally. The pharmacokinetics of amoxicillin is not affected in the fasted or fed state, but absorption of clavulanate potassium is more when taken with food. Clavulanate has elimination t1/2 of 1 hour and equals that of amoxicillin.
About 25 - 40% of clavulanic acid and more than half of amoxicillin are eliminated through urine without undergoing any metabolism. Clavulanic acid is eliminated mainly by glomerular filtration and its excretion is not affected by probenecid. Also, it is largely hydrolysed and decarboxylated before excretion, while amoxicillin is primarily excreted unchanged by tubular secretion.
Thus the pharmacokinetics of amoxicillin and clavulanic acid are largely similar and neither appears to affect the other to any great magnitude.
Microbiology:
Amoxicillin is degraded by beta lactamases and its spectrum of activity is extended by use of clavulanic acid. Clavulanic acid reverses resistance to amoxicillin in beta-lactamase-producing strains of species otherwise sensitive and also augments the activity of amoxicillin against numerous species not normally considered sensitive. It is effective against following microorganisms:
Gram-Positive Aerobes:
Staphylococcus aureus
Gram-Negative Aerobes:
H. influenzae
Escherichia coli
Klebsiella
Moraxella catarrhalis
Enterobacter species
Gram-Positive Aerobes:
Streptococcus pneumoniae
Streptococcus pyogenes
Enterococcus faecalis
Staphylococcus epidermidis and saprophyticus
Gram-Negative Aerobes:
Neisseria gonorrhoeae
Proteus mirabilis
Eikenella corrodens
Anaerobic Bacteria:
All Bacteroides species
Fusobacterium species
Peptostreptococcus species
Indications and Usage
Due to the hazard of cholestatic jaundice, amoxicillin/clavulanic acid is not a treatment of choice for common bacterial infections. It should not be given for more than 14 days and should be prescribed only for infections caused by resistant microorganisms which produce beta-lactamase. It may be considered for:
- Otitis media, tonsillitis, sinusitis
- Bronchopneumonia
- Acute exacerbations of chronic bronchitis
- Urinary-tract infections, (recurrent or complicated), it is not indicated for prostatitis
- Abortion resulting in infection, intra-abdominal and pelvic or puerperal sepsis.
- Severe dental abscess and cellulitis.
- Gonorrhoea (including PPNC) single dose amoxicillin 3 g + clavulanic acid 0.5 g + probenecid 1 g is highly curative.
- Empirical antibiotic therapy for hospital acquired infections.
Contraindications and Warnings
Amoxicillin/clavulanic acid is contraindicated in persons having prior history of allergic reactions to any of the penicillin group of antibiotics.
Prior history of cholestatic jaundice or liver dysfunction seen with its use is also a contraindication. Hepatic toxicity associated with its use is usually reversible.
Precautions
The likelihood of super-infections must be known during therapy.
Erythematous rashes may develop on the skin particularly in persons suffering from infectious mononucleosis and who take ampicillin group of antibiotics including amoxicillin.
Drug-drug Interactions:
Reduction of tubular secretion in kidney of amoxicillin is caused by probenecid. Allopurinol may reduce renal tubular secretion of amoxicillin thus increasing its serum levels.
Concurrent use of amoxicillin/Clavunate may lead to oral contraceptive failure due to decreased plasma levels of hormones.
Interactions with laboratory tests: If Fehling’s or Benedict’s Solution for urine glucose estimation false positive results may be seen if patient is taking amoxicillin.
Carcinogenesis, teratogenic effects, mutagenic effect, and fertility impairment: Animal studies have not demonstrated any of these.
Paediatric Use: Excretion of amoxicillin may be delayed as renal function is not well developed in neonates and young infants. Dose should be adjusted in paediatric patients younger than 3 months.
Nursing Mothers: Caution should be exercised as ampicillin-class antibiotics are excreted in the milk.
Adverse reactions
Amoxicillin/clavunate is usually well tolerated. The most frequently reported adverse effects were diarrhea/loose stools, nausea, skin rashes and urticaria, vomiting and vaginitis. Other less frequently reported reactions were abdominal discomfort, flatulence, headache, slight thrombocytosis and increased prothrombin time in patients receiving anticoagulants concomitantly. Only < 3% of patients discontinued therapy due to drug-related side effects.
Rarely hepatic dysfunction, including hepatitis and cholestatic jaundice, increases in serum transaminases, serum bilirubin and/or alkaline phosphatase, has been reported. It has been reported more commonly in the elderly, in males, or in patients on prolonged treatment. It appeared that clavulanic acid was probably responsible. Risk of acute liver injury was about 6 times greater with the combination than with amoxicillin alone.
Renal: Rarely interstitial nephritis, haematuria and crystalluria have been reported.
Other adverse effects are similar to those of amoxicillin.