 Français
Français Antibiotic Drugs
Ceftaroline Fosamil
Ceftaroline is a novel, broad-spectrum fifth generation cephalosporin. It is approved by FDA in October 2010.
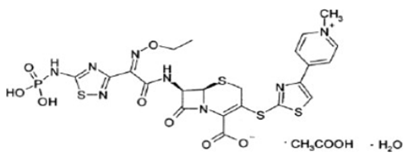
Chemical structure
Its molecular formula is C22H21N8O8PS4.C2H4O2.H2O and MW is 604.7.The chemical structure is:
Mechanism of action
Mechanism of action of ceftaroline is bit different from penicillin. It inhibits penicillin binding protein-2a causing inhibition of bacterial cell wall synthesis, lack of bacterial cell wall results in death due to lysis of bacteria.
Pharmacokinetics
Ceftaroline's pharmacokinetic profile is comparable to other cephalosporins. Ceftaroline fosamil is a prodrug.
Antimicrobial spectrum
Ceftaroline is highly effective against gram-positive cocci including MRSA, gram-negative bacilli. It has activity against few anaerobes and not active against gram-negative cocci and gram-positive bacilli.
Indications and uses
Ceftaroline is used for the treatment of community-acquired pneumonia, acute bacterial skin and skin structure infections.
Administration and dosage
It is available as ceftaroline fosamil monoacetate monohydrate and the dosage is expressed in terms of anhydrous ceftaroline fosamil.
| Condition | Dose (IV) |
| Community-acquired Pneumonia | 600 mg/12 hourly for 5–7 days. |
| Skin and Skin Structure Infections | 600 mg/12 hourly for 5–14 days |
The duration of treatment depends on the site and severity of infection and patient’s clinical and bacteriologic progress.
Precautions, contraindications and warnings
Ceftaroline is contraindicated in patients with prior history of hypersensitivity to ceftaroline or other cephalosporins.
Adverse Effects
Side effects of ceftaroline are diarrhea, nausea, vomiting, constipation, headache, rash, pruritus, increased transaminases, anemia, eosinophilia, neutropenia, thrombocytopenia, bradycardia, palpitations, abdominal pain, fever, hepatitis, hyperglycemia, hyperkalemia, dizziness, convulsion, renal failure and urticaria.
Technical Description on Ceftaroline Fosamil
Ceftaroline is a novel, broad-spectrum fifth generation cephalosporin.
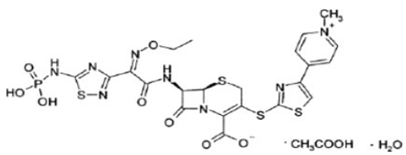
Chemical structure
It is a prodrug and derived from the fourth-generation cephalosporin, cefosopran. Its molecular formula is C22H21N8O8PS4.C2H4O2.H2O and MW is 604.7.The chemical structure is:
Preparations available
Parenteral – 400 mg and 600 mg /vial
Mechanism of action
The mechanism of action of Ceftaroline is analogous to that of other β-lactam antibiotics. It binds to penicillin binding proteins (PBPs) in both gram-negative and positive bacteria and prevents their capability to act as transpeptidases in bacterial cell wall formation. Ceftaroline has good affinity for penicillin binding protein 1-3 in Staphylococcus aureus, with limited or no affinity for penicillin binding protein -4. Along with this it also preserves activity against penicillin binding proteins in gram-positive bacteria which have undergone mutation. An attachment site is opened in the penicillin binding protein 2a due to a structural alteration by ceftaroline. After attachment ceftaroline produces an intermediate complex of the enzyme known as acyl which performs the main function of inhibition.
Microbiology
Ceftaroline is active against:
Gram positive bacteria
- S. aureus (which includes VRSA, MRSA and daptomycin-resistant strains )
- Coagulase-negative staphylococci
- Pneumococci (even multidrug resistant )
- S. pyogenes
- Viridans streptococci
- S. agalactiae and dysgalactiae
E. faecium are not sensitive but it has some effect against E. faecalis.
Gram negative bacteria
- E. coli
- Proteus mirabilis
- Haemophilus influenzae and parainfluenzae
- Moraxella catarrhalis
- Klebsiella pneumoniae and oxytoca
- Morganella morganii
- Pasteurella multocida
- Citrobacter koseri and freundii
- Enterobacter cloacae and aerogenes
Pseudomonas aeruginosa is resistant.
Resistance
Bacteria of the gram negative group which form extended spectrum beta-lactamases from the CTX-M, TEM, or SHV genes are resistant to ceftaroline. Also gram negative bacteria which express metallo-beta-lactamases of class B or C and serine carbapenemases like KPC are resistant to ceftaroline. Some cross resistance is seen with ceftaroline to cephalosporins.
The gram positive bacteria have fewer propensities to develop resistance to ceftaroline. Resistance to ceftaroline in gram negative bacteria is mainly due to altered permeability and efflux of the drug from the bacterial cell.
Pharmacokinetics
The pharmacokinetic profile of Ceftaroline is predictable and comparable to other renally excreted cephalosporins. Ceftaroline fosamil is a prodrug; it is rapidly dephosphorylated in vivo to ceftaroline by a plasma phosphatase predominantly during IV infusion. In addition, the beta lactam ring of ceftaroline is hydrolyzed to an inactive metabolite known as ceftaroline M-1.
Not much information exists about tissue distribution but animal data suggests that ceftaroline is distributed into kidneys, skin, and lungs. The t1/2 in adults is 2.7 hours. The plasma protein binding is about 20%. Ceftaroline is not a substrate of CYP isoenzymes. Ceftaroline is excreted through the kidney. More than half of ceftaroline is excreted through the urine without any metabolism.
Therapeutic uses
- Community-acquired Pneumonia
It is used for the therapy of community-acquired pneumonia caused by sensitive microorganisms mentioned above.
- Infections of skin and appendages
It is also used for the therapy of infections of skin and appendages caused by sensitive bacteria as mentioned above.
Dosage
It is available as ceftaroline fosamil monoacetate monohydrate and the dosage is expressed in terms of anhydrous ceftaroline fosamil.
| Condition | Dose (IV) |
| Community-acquired Pneumonia | 600 mg/12 hourly for 5–7 days. |
| Infections of skin and appendages | 600 mg/12 hourly for 5–14 days |
Special Populations
- Liver dysfunction
Not much data is available but hepatic impairment is not expected to have a clinically significant effect on systemic clearance of the drug.
- Kidney dysfunction
Dosage modification is required in persons with kidney dysfunction.
- Pregnancy
It is pregnancy category B drug.
- Lactation
It is not known whether ceftaroline is secreted during lactation.
- Children
The efficacy and safety of ceftaroline is not recognized in patients <18 years of age.
Contraindications
Ceftaroline is contraindicated in patients with prior history of hypersensitivity to ceftaroline or other cephalosporins.
Warnings
- Clostridium difficile-associated enterocolitis
There is a risk of development of enterocolitis due to clostridium difficile. Also super-infections can develop due to modification of the intestinal flora due to telavancin.
- Haematological
The coombs test may be turn from negative to positive in some patients with or without coexistent haemolytic anemia which is rarely seen.
If patients develop anemia on therapy with ceftaroline it should be taken as drug induced haemolytic anaemia unless otherwise proved and appropriate diagnostic tests should be carried out.
Drug interactions
Ceftaroline does not inhibit CYP1A1, 1A2, 2A6, 3A4, 2C9, 2C19, 2B6, 2C8, 2D6, 2E1, or in vitro; does not induce CYP1A2,2B6, 3A4/5, 2C8, 2C19, 2C9. Pharmacokinetic interactions with the drugs metabolized by these isoenzymes are improbable.
Synergism is exhibited by aztreonam, meropenem and amikacin along with ceftaroline against ESBL-producing K. pneumoniae and E. coli, pseudomonas aeruginosa and AmpC-derepressed E. cloacae, and Ps. aeruginosa.
Carcinogenic potential, Mutagenic potential and impaired fertility
Animal studies have not proved any of these with telavancin.
Adverse Effects
The common adverse effects are:
- Loose stools, constipation and vomiting
- Skin rashes, increased itching, decreased serum potassium levels and headache
- Increased hepatic enzymes, phlebitis
Other less commonly observed adverse Reactions are:
- Anemia, increased eosinophil count, decreased neutrophil count and decreased platelet count
- Hepatitis
- Increased blood glucose levels, increased serum potassium levels
- Giddiness, seizures
- Kidney failure
- Decreased heart rate ,Palpitations
- Pain in the abdomen
- Fever